News Story
Using Brillouin Microscopy, Scarcelli Aims to Shed Light on How Tumor Cells Metastasize

For years, Fischell Department of Bioengineering (BIOE) Assistant Professor Giuliano Scarcelli has worked to develop a novel optical microscopy technique that could be used to help scientists measure the mechanical properties—such as stiffness—of biological tissue and cells without contact.
Now, with support from a three-year grant totaling more than $1 million in funding from the Innovative Molecular Analysis Technologies program of the National Cancer Institute of the National Institutes of Health, Scarcelli and members of his Optics Biotech Lab are working to use the technique, known as Brillouin optical microscopy, to shed light on how and why tumor cells metastasize to other parts of the body.
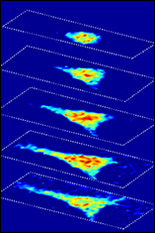
Using Brillouin light scattering, a process that occurs when light interacts with density fluctuations in a medium, Scarcelli and his team are able to measure how mechanical properties of a cell change over time in their 3-D microenvironments. These spontaneous fluctuations are driven by collective acoustic vibrational modes, and thus depend on the local mechanical properties within a sample. In this way, Brillouin microscopy yields invaluable information on the viscoelastic characteristics of cells – and does so at a microscopy resolution no other non-contact technique can match.
“Gold-standard techniques require contact, therefore, they are inherently limited to providing global averages of cell properties,” Scarcelli said. “With Brillouin microscopy, we can characterize the mechanical properties within a cell and we can do that in experimental situations where there is no physical access to the cell, such as within 3-D environments that mimic in vivo conditions.”
Scientists have long recognized that the mechanical interplay between a cell and its surrounding microenvironment influences tumor progression, malignancy transformation, and metastasis. In order for cancer to progress from one area of the body to another, a tumor cell leaves the original cancer site through a complex process known as the metastatic cascade before a new tumor forms at a secondary organ site.
“During the metastatic cascade, tumor cells face very difficult mechanical challenges; for example, they may squeeze through constrictions smaller than the cell’s size,” Scarcelli said. “Yet, some cells are able to overcome these challenges. Hopefully, we'll now be able to see what type of mechanical machinery metastatic cells have to put in place to do this.”
Understanding how a tumor cell accomplishes the steps of the metastatic cascade could help bioengineers develop improved diagnostic tools or improved drug screening platforms.
Scarcelli specializes in biophotonics, with strong emphasis on optical sciences and technology development. Prior to joining the department in 2014, he served as an instructor with the Harvard Medical School and Wellman Center for Photomedicine at Massachusetts General Hospital. He is the inventor in four patents, all licensed to industry, and his work with Brillouin microscopy earned him the Tosteson Postdoctoral Fellowship Award, an NIH K25 Career Development Award, and a Young Investigator Award from the Human Frontier Science Program.
Research reported in this article was supported by the National Cancer Institute of the National Institutes of Health under Award Number R33CA204582. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Published June 28, 2016