News Story
The Gel with the Secret Center
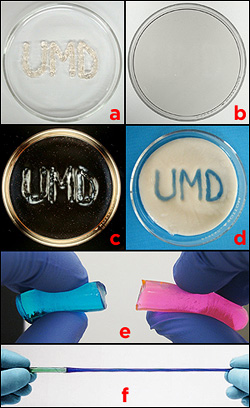
Hybrid gels. a) A message is written with the first liquid pre-gel, thickened with Laponite clay particles, on a petri dish, and the second is poured around it. b) After polymerization, the result is a single, clear gel. c) The area containing Laponite particles refract polarized light differently than those that don't, revealing the message. d) In a similar experiment, the outer gel was heat sensitive. The Laponite-laced gel was not, and remained clear when heated, revealing the message. e) Hybrid gels made by polymerizing one, pouring in a second, and then polymerizing it, result in a weak bond between the layers. f) The Raghavan Group's technique results in a strong bond with very little mixing of the two pre-gels. In this example, the hybrid gel does not tear at the junction when stretched, and it exhibits two different mechanical properties: the blue side, a physically crosslinked polymer, is more flexible than the green side, a chemically crosslinked polymer.
The group, led by the lab’s director, Department of Chemical and Biomolecular Engineering (ChBE) professor Srinivasa Raghavan, recently published a paper describing the gel in Macromolecules. The work was also featured in the New Scientist’s “One Per Cent” column.
The Raghavan Group isn't the first to combine gels with different properties, but team members believe they are the first to overcome a significant obstacle to doing it well.
"People have tried layering different gels together by polymerizing the first, layering the second in its liquid pre-gel state on top, then polymerizing that one, and so on, sort of like layering Jell-O," explains Stephen Banik (M.S. ’12, chemical engineering), who first-authored the paper and wrote his thesis on the gel. "The problem is the layers are only weakly hooked together where they interface. If you apply just a little bit of stress to them, they come apart."
The key to creating strong bonds, he says, is to polymerize two or more liquid pre-gels at the same time, allowing them to interpolymerize across an interface. The challenge is to do it without allowing the pre-gels to mix.
The secret of his gels' success is Laponite®, an inorganic clay used to control the viscosity of many water-based products such as paint and cosmetics. Banik found he could put two liquid pre-gels next to or on top of each and polymerize them simultaneously, without mixing, as long as one of them had been thickened with Laponite. The difference in viscosity allowed each material to retain its special properties while gelling and bonding. The result was a single, rugged gel that did not tear apart or rupture where the two zones met.
Banik created two kinds of gels that revealed hidden messages inside under different circumstances. In both cases he used cake-decorating bags to write the letters “UMD” in a Laponite-thickened pre-gel on a shallow dish. He then carefully poured a second, liquid pre-gel around it. The first pre-gel’s thickness prevented it from dissolving into the second, like a line of toothpaste surrounded by water. After polymerization, the dish appeared to contain a single, clear gel, but different areas responded to different stimuli.
One sample, when heated, turned opaque where the second pre-gel had been poured, revealing the message as clear letters. The other’s zones had different optical properties: the Laponite particles in the initials “UMD” refracted polarized light differently than the gel around it, making them visible.
The same technique was also used to make hybrid gels containing different levels of flexibility, ones that shrank and expanded as they retained and lost water at different rates, and ones that selectively absorbed dyes.
With additional development, hybrid gels like these could be used in industrial or biomedical products. Potential applications include coatings that respond to changes in temperature, conditional or extended release capsules for drugs, biomimetic devices, tissue engineering, and optical computing.
For More Information:
"A New Approach for Creating Polymer Hydrogels with Regions of Distinct Chemical, Mechanical, and Optical Properties." Stephen J. Banik, Neville J. Fernandes, Peter C. Thomas, and Srinivasa R. Raghavan. Macromolecules 2012, 45, 5712−5717.
"Gel mixture lets you hide a secret message in goo." Jacob Aron. New Scientist, 15:13, 13 July 2012.
Visit the Complex Fluids and Nanomaterials Group web site »
Published September 25, 2012









